Pipeline
Therapeutic field & Development
Proprietary Technology
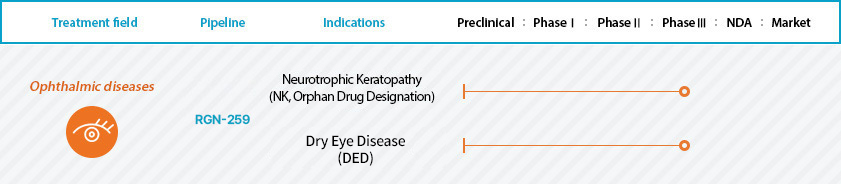
RGN-259 - Most Advanced, Preservative-Free, Single Unit Dose
- RGN-259 ophthalmic solution is formulated as thymosin beta 4 preservative-free eye drops for the treatment of both dry eye disease and neurotrophic keratopathy. Thymosin beta 4 (Tβ4) is a synthetic copy of the naturally-occurring 43-amino acid peptide that is found in all tissues and in all nucleated cells. In the eye, Tβ4 promotes corneal epithelial cell migration, decreases both inflammation and apoptosis, and accelerates both repair and regeneration.
- RGN-259, our most advanced product, is designed and engineered to meet the needs of the current ophthalmic therapeutic market and for the convenience of patients who need to use an eye product daily. RGN-259 has been developed as a single unit dose based on both proprietary manufacturing technology and cutting-edge blow-fill-seal technology at an experienced cGMP facility according to strict cGMP standards. ReGenTree currently conducting the 2nd and 3rd phase III clinical trial(SEER-2, SEER-3) for the treatment of Neurotrophic Keratopathy in the United States and EU. ReGenTree also conducted the 3rd Phase III clinical trial(ARISE-3) for the treatment of Dry Eye Disease and planning the 4th phase III clinical trial(ARISE-4) for the treatment of Dry Eye Disease.
Market Territory

Please contact HLB Therapeutics if you are interested in partnering (other than China, Hong Kong, Taiwan).

